As
we all know about aerosols, let me first start with the question that what are
these particles? Do they have direct effect on climate change? Or they
indirectly affect it?
Here
we go; Aerosols are the tiniest particles which remains suspended in the
atmosphere, most specifically in the lower atmosphere or troposphere. Different
specialists describe the particles based on shape, size, and chemical
composition.
Climatologists
typically use another set of labels that speak to the chemical composition. Key
aerosol groups include sulfates, organic carbon, black carbon, nitrates,
mineral dust, and sea salt. In practice, many of these terms are imperfect, as
aerosols often clump together to form complex mixtures. It’s common, for
example, for particles of black carbon from soot or smoke to mix with nitrates
and sulfates, or to coat the surfaces of dust, creating hybrid particles (1).
These
particles keep moving in atmosphere and interact with atmosphere in two ways; directly
and indirectly. Direct interaction with atmosphere leads to generate the blue
sky, and another colors in different events, like reddish and yellowish color
at the time of sunset and sunrise, we all know that this is due to scattering
of incoming sun's radiation in atmosphere. In true words these aerosols are
capable of reflecting back the sun's radiation in a large extent which leads to
decrease in the temperature or truly speaking retards to enter it into the our
atmosphere. Whatever we talked about was only the thing which can observe with
our senses, and we technically admire it. But the indirect interaction is quit
complex phenomena, hard to understand, but it's true too, that aerosols affects
the formation of clouds in lower atmosphere. The amount of aerosol present in
lower atmosphere, modifies the reflectivity of clouds to sun light, also
changes the size of cloud in lower atmosphere.
 |
| Aerosol Interaction with cloud and ozone deterioration |
Aerosols
are also responsible for deterioration of stratospheric ozone, which is due to its
chemically reactive property. How this happens it’s a complex chemistry, what
we can understand is that it chemically react with the atmospheric water which
may be available as water vapor, this leads to formation of chemically active
clouds which generates the highly active chlorine which react with the ozone.
Well this is a complex thing. Another thing is to notice that what are the
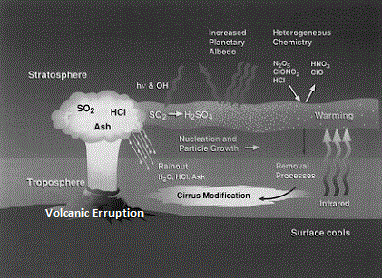
other impacts of aerosols? Aerosol may cause acid rain due to the presence of
chemically active particles which forms acids; these may be SO2 or HCL which is
generally erupted by volcanic movements. These aerosols when comes in contact
with water, rains as acid rain mainly in the form of hydrochloric acid and
sulfuric acid.
Now
question rises that where from these aerosols come, actually aerosols are the
part of our nature hence the atmosphere too. Nature itself carries these
particles in suspension, but some of the aerosols are added to our atmosphere
by man's activities.
The
main sources of aerosols are:
1.
Desert Dust
2.
Volcanic Eruption added
3.
Man Made
As
i mentioned above that nature itself carry the aerosols, about 90% of by mass
of aerosols are naturally originated and added to the atmosphere. Volcanic
eruption is the one of the major natural phenomena which introduces huge amount
of ash into air, SO2 and other gases which yields sulfates. Naturally occurring
forest fires, introduces lot of organic carbon. Some plants produce gases that
react with other substances in the air to yield aerosols, such as 'smoke' in
the Great Smoky Mountains of United States. Likewise in the ocean, some types
of micro-algae produces a sulfurous gas called dimethyl sulfide that can be
converted into sulfates in the atmosphere(1). Sea salts which vaporized with
water, most abundant aerosols. Sand particles are also present in huge amount
added by the high speed winds.
Rest
amount of aerosols are considered to be added by anthropogenic activities,
having a great variety. Mainly these are added by the industrial activities,
due to fossil fuel combustion which induces large amount of sulfur-di-oxide.
Other activities like consuming farm waste, preparation of farm land yields
smoke, which is a source of organic carbon.
Automobiles
incinerators, smelters, and power plants are prolific producers of sulfates,
nitrates, black carbon and other particles. Deforestation overgrazing drought
and excessive irrigation can alter the land surface, increasing the rate at
which dust aerosols enter the atmosphere. Even indoors, cigarettes, cooking
stoves, fireplaces, and candles are source of aerosols.
Aerosols and sunlight:
Different
type of aerosols scatters or absorbs sunlight in different proportions,
depending upon the physical properties (shape, size etc.). It is a direct
effect of aerosols, which directly deal with earth’s radiative field. Mostly
aerosols reflect back the sunlight depending upon their color and composition,
but some darker aerosols absorb it too.
Pure
sulfates and nitrates reflect nearly all radiation they encounter, cooling the
atmosphere. Black carbon, in contrast, absorbs radiation readily, warming the
atmosphere but also shading the surface. Organic carbon, sometimes called brown
carbon or organic matter, has a warming influence on the atmosphere depending
on the brightness of the underlying ground. Dust impacts radiation to varying
degrees, depending on the composition of the minerals that comprise the dust
grains, and whether they are coated with black or brown carbon. Salt particles
tend to reflect all the sunlight they encounter (1).
If
amount of aerosols suddenly increased in the atmosphere; than it cause sudden
cooling effect. Aerosols, particularly black carbon, can alter reflectivity by
depositing a layer of dark residue on ice and other bright surfaces. In the
Arctic especially, aerosols from wildfires and industrial pollution are likely
hastening the melting of ice.
Scientists
believe the cooling from sulfates and other reflective aerosols overwhelms the
warming effect of black carbon and other absorbing aerosols over the planet.
Models estimate that aerosols have had a cooling effect that has counteracted
about half of the warming caused by the build-up of greenhouse gases since the
1880s. However, unlike many greenhouse gases, aerosols are not distributed
evenly around the planet, so their impacts are most strongly felt on a regional
scale.
Aerosols and clouds:
Aerosols
play a critical role in the process of cloud formation. In fact, most clouds formed
due to the presence of aerosols which serves as the tiny “seeds,” called cloud
condensation nuclei.
Natural
aerosols—often sulfates, sea salt or ammonium salts—are the most common
condensation nuclei in pristine environments. Polluted air, in contrast,
usually contains much higher concentrations of water-soluble particles, which
means pollution-rich clouds tend to have more numerous, but smaller, droplets.
The small droplets make polluted clouds look brighter than they would otherwise
be. Just as many bits of crushed ice give light more surfaces to reflect
off—appearing brighter than a solid cube of ice—if the water in a cloud is
divided into a larger number of smaller droplets, it will scatter more light
and become more reflective(1).
Brighter
clouds, in turn, block sunlight from reaching Earth’s surface, shading the
planet and producing net cooling. This cloud brightening effect—called the
“cloud albedo effect”—may have a big impact on the climate, though only in
recent years has it been possible to start quantifying the effect.
This
impact of aerosols is clearly visible in ship tracks, bright streaks in marine
clouds that look like airplane contrails. In the absence of ships, sea salt
particles and the natural sulfates produced by phytoplankton seed most marine
clouds. However, the exhaust from ship smokestacks make trails of sulfates and
other aerosols that form long, bright clouds.
Concluding part of this text is that presence of aerosols in lesser of higher amount, modifies the climatic changes over an region.
No comments:
Post a Comment